Translate:
We are the Leaders in Immuno-Oncology & Liquid Biopsy Testing.
Clinical Relevance of Genomics in Cancer Medicine
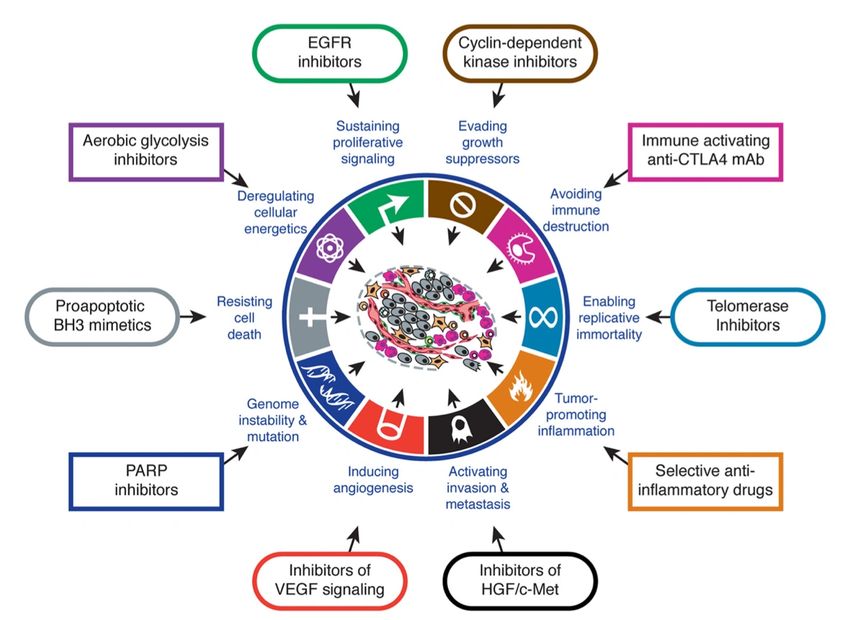
- Genomic assays that enable the characterization of the somatic and germline defects in individual tumour samples are increasingly being used in clinical diagnostics as a means of identifying therapeutic options.
- Genomic methods can reveal individual targetable alterations, mutational load, complex mutation signatures, and tumour-specific antigens, which might inform the utilization of targeted therapies, immune-checkpoint inhibitors, and personalized anticancer vaccines.
- The occurrence of shared targetable alterations across diverse tumour types has prompted new paradigms in the application of genomic profiling and the design of clinical trials.
These Assays Increasingly Provide Information
That is Pertinent to Clinical Cancer Care.
We Offer Niche Tests & Technologies
Liquid Biopsy
Multi - Gene Testing
Immuno Oncology
Circulating Tumor DNA (ctDNA)
Circulating Tumor Cell Detection (CTC)
Our Liquid Biopsy services can assist your patients in:
- Performing tumor profiling
- Surveillance of tumors and their response to therapy
- Monitor heterogeneity
- Testing for minimal residual disease (MRD)
- Helping predict early relapse
Testing using Peripheral Blood based liquid bio
Circulating Tumor DNA (ctDNA)
Circulating Tumor Cell Detection (CTC)
Our Liquid Biopsy services can assist your patients in:
- Performing tumor profiling
- Surveillance of tumors and their response to therapy
- Monitor heterogeneity
- Testing for minimal residual disease (MRD)
- Helping predict early relapse
Testing using Peripheral Blood based liquid biopsies is more sensitive than other diagnostic testing methods like bone marrow taps, which are invasive and painful for patients.
Peripheral Blood based Tumor DNA Detection Tests are available for:
- Lung Cancer - Screens 11 Lung Cancer Related "ACTIONABLE" Genes in peripheral Blood: ALK, BRAF, EGFR, ERBB2, KRAS, MAP2K1, MET, NRAS, PIK3CA, ROS1, and TP53. Review matching drugs for each mutation.
- Colon Cancer - Screens 3 Colon Cancer Related "ACTIONABLE" Genes in peripheral Blood: BRAF, NRAS, KRAS. Review matching drugs for each mutation
Contact Us to order a liquid Biopsy Test. medical diagnostic service blood test for cancer nsclc
Immuno Oncology
Multi - Gene Testing
Immuno Oncology
Solid Tumors
Leukemia's and Lymphoma
Immuno-Oncology (I-O) is the investigation of innovative approaches that aim to harness the body’s natural immune response to fight cancer.
Immuno-Oncology testing helps determine which patients are good candidates for immunotherapy. Star Bioscience Laboratories is a leader in precision diagnostics and i
Solid Tumors
Leukemia's and Lymphoma
Immuno-Oncology (I-O) is the investigation of innovative approaches that aim to harness the body’s natural immune response to fight cancer.
Immuno-Oncology testing helps determine which patients are good candidates for immunotherapy. Star Bioscience Laboratories is a leader in precision diagnostics and is at the forefront of immunotherapy testing.
Tumor Mutation Burden (TMB): This genomic biomarker is designed to predict response to checkpoint inhibitor immunotherapies targeting the PD-1 and PD-L1 proteins. Studies in lung, melanoma and bladder cancers showed that objective response to checkpoint immunotherapy was predicted by the presence of high TMB.
Microsatellite Instability (MSI) and Mismatch Repair (MMR): KEYTRUDA® was approved by the FDA for the treatment of certain patients with metastatic solid tumors that have been identified as being MSI-high or mismatch repair deficient (dMMR)1. OPDIVO® is FDA-approved for certain colorectal cancer patients with MSI-high or dMMR2.
PD-L1: This immunohistochemical assay detects the level and expression sites of PD-L1 protein. As a companion or complementary diagnostic for certain tumors and indications, it helps identify patients for treatments including KEYTRUDA, OPDIVO, TECENTRIQ® and IMFINZI™.
Contact Us to order an IO Test.
Multi - Gene Testing
Multi - Gene Testing
Multi - Gene Testing
Star Bioscenices has developed Next Generation Sequencing (NGS) testing for all cancers.
Our approach is to serve your patients with affordable Cancer Specific - Next Generation Sequencing Multi-Gene Panels which screen 10, 50 to > 400 genes in All Solid Tumors, including:
Star Bioscenices has developed Next Generation Sequencing (NGS) testing for all cancers.
Our approach is to serve your patients with affordable Cancer Specific - Next Generation Sequencing Multi-Gene Panels which screen 10, 50 to > 400 genes in All Solid Tumors, including:
- Lung Cancer
- Breast Cancer
- Colon Cancer
- Pancreatic Cancer
- Prostate Cancer
- Glioblastoma
- Sarcoma's
- Melanoma
- Leukemia & Lymphoma's
Tests Reports include analysis from DNA, RNA sequencing along with Pharmacogenomics.
Contact Us to order a NGS Test.
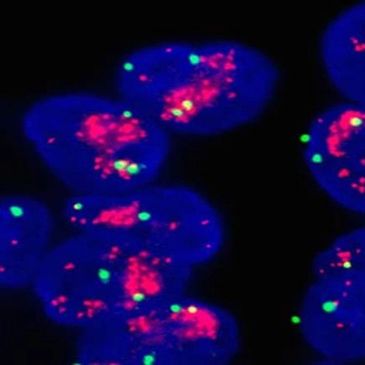
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH) can help identify subtle or sub-microscopic structural rearrangements, variant chromosomes, and low-frequency abnormalities not readily detectable by classic cytogenetics.
FISH incorporates three technologies – cytogenetics, DNA hybridization, and fluorescent microscopy – to provide a uniquely inf
Fluorescence in situ hybridization (FISH) can help identify subtle or sub-microscopic structural rearrangements, variant chromosomes, and low-frequency abnormalities not readily detectable by classic cytogenetics.
FISH incorporates three technologies – cytogenetics, DNA hybridization, and fluorescent microscopy – to provide a uniquely informative combination of resolution and breadth.
FISH is also called Molecular Cytogenetics.
Star Biosciences provides our clients a full menu of panels and probes with flexible ordering options to enable rapid and cost-effective characterization of solid tumors like Lung Cancer and Breast Cancer, leukemias, lymphomas, and myeloid disorders.
Contact Us to order a FISH Test.
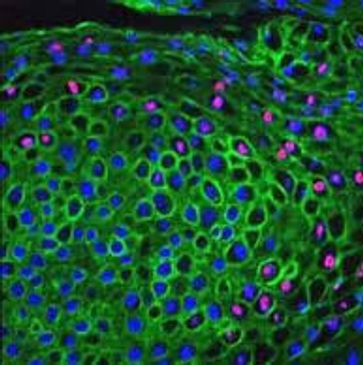
Immunohistochemistry (IHC)
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH)
Immunohistochemistry (IHC) is a core cancer diagnostics testing method that is playing an increasingly important role in precision medicine.
It combines immunological and biochemical techniques to identify specific components of a tissue. IHC uses a building-block system of antibodies, conjugates, and chromogens (color reagents) for detec
Immunohistochemistry (IHC) is a core cancer diagnostics testing method that is playing an increasingly important role in precision medicine.
It combines immunological and biochemical techniques to identify specific components of a tissue. IHC uses a building-block system of antibodies, conjugates, and chromogens (color reagents) for detection.
A significant advantage of IHC over other methods is that IHC allows the distribution and density of components to be visualized in relation to each other and to the rest of the tissue sample.
Contact Us to order a IHC Test.
Molecular Tests & Heme Onc
Fluorescence in situ hybridization (FISH)
Molecular Tests & Heme Onc
We makes it easy for hemepaths and hem/oncs to solve medical problems in two main ways.
- First, our menu allows physicians to choose the level of detail and investigation each patient requires for diagnosis, prognosis, and/or therapy selection. From simple orders for standard guideline-recommended markers to multi-parameter assays incorpor
We makes it easy for hemepaths and hem/oncs to solve medical problems in two main ways.
- First, our menu allows physicians to choose the level of detail and investigation each patient requires for diagnosis, prognosis, and/or therapy selection. From simple orders for standard guideline-recommended markers to multi-parameter assays incorporating novel discoveries,
- Star Biosciences provides clear, cost-effective, and efficient testing solutions that fit today’s needs.
These are some of the most frequently ordered heme tests on our menu.
- Molecular Testing - individual Markers and Panels
- Cytogenetics
- Flow-Cytometry
Contact Us to order a Molecular or Heme-Onc Test.
Contact Us
India: Contact Customer Service Representatives @ Tel: 9794048252
SE-Asia:
Drop us a line and our Customer Service will contact you.
Africa:
Drop us a line and our Customer Service will contact you.
FDA Approved Precision Drugs - Solid Tumors
DRUGS FOR SOLID TUMORS
Ado-trastuzumab emtansine (Kadcyla)
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Target: EGFR (HER1/ERBB1), HER2 (ERBB2/neu)
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR exon 19 deletions or exon 21 substitution (L858R) mutations)
- FDA Approved Indication:
- Renal cell carcinoma
- Melanoma.
- Target: ALK
- FDA Approved Indication:
- Non-small cell lung cancer (with ALK fusion)
- Target: PD-L1
- FDA Approved Indication:
- Urothelial carcinoma
- Non-small cell lung cancer
- Target: PD-L1
- FDA Approved Indication:
- Merkel cell carcinoma
- Urothelial cancer
- Target: KIT, PDGFRβ, VEGFR1/2/3
- FDA Approved Indication:
- Renal cell carcinoma
- Target: BAFF
- FDA Approved Indication:
- Lupus erythematosus
- Target: VEGF ligand
- FDA Approved Indications:
- Cervical cancer
- Colorectal cancer
- Fallopian tube cancer
- Glioblastoma
- Non-small cell lung cancer
- Ovarian cancer
- Peritoneal cancer
- Renal cell carcinoma
- Target: ABL
- FDA Approved Indication:
- Chronic myelogenous leukemia (Philadelphia chromosomepositive)
- Target:ALK
- FDA Approved Indication:
- Non-small cell lung cancer (ALK+)
- Target: FLT3, KIT, MET, RET, VEGFR2
- FDA Approved Indication:
- Medullary thyroid cancer
- Renal cell carcinoma
- Target: ALK
- FDA Approved Indication:
- Non-small cell lung cancer (with ALK fusion)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Colorectal cancer (KRAS wild type),
- Squamous cell cancer of the head and neck
- Target: MEK
- FDA Approved Indication:
- Melanoma (with BRAF V600E or V600K mutation)
- Target: ALK, MET, ROS1
- FDA Approved Indication:
- Non-small cell lung cancer (with ALK fusion or ROS1 genealteration)
- Target: BRAF
- FDA Approved Indication:
- Target: RANKL
- FDA Approved Indication:
- Giant cell tumor of the bone
- Target: B4GALNT1 (GD2)
- FDA Approved Indication: Pediatric neuroblastoma
- Target: PD-L1
- FDA Approved Indication:
- Urothelial carcinoma
- Non-small cell lung cancer
- Target: IDH2
- FDA Approved Indication:
- Acute myeloid leukemia (with IDH2 mutation)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR exon 19 deletions or exon 21 substitution (L858R) mutations)
- Pancreatic cancer
- Target: mTOR
- FDA Approved Indication:
- Pancreatic, gastrointestinal, or lung origin neuroendocrine tumor
- Renal cell carcinoma
- Nonresectable subependymal giant cell astrocytoma associated with tuberous sclerosis
- Breast cancer (HR+, HER2-)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR exon 19 deletions or exon 21 substitution (L858R) mutations)
- Target: BTK
- FDA Approved Indication:
- Mantle cell lymphoma
- Chronic lymphocytic leukemia
- Waldenstrom's macroglobulinemia
- Target: PI3Kδ
- FDA Approved Indication:
- Chronic lymphocytic leukemia
- Follicular B-cell non-Hodgkin lymphoma
- Small lymphocytic lymphoma
- Target: KIT, PDGFR, ABL
- FDA Approved Indication:
- GI stromal tumor (KIT+)
- Dermatofibrosarcoma protuberans
- Multiple hematologic malignancies including Philadelphia chromosome-positive ALL and CML
- Target: CTLA-4
- FDA Approved Indication:
- Melanoma
- Renal cell carcinoma
- Target: Proteasome
- FDA Approved Indication: Multiple Myeloma
- Target: HER2 (ERBB2/neu), EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Target: VEGFR2
- FDA Approved Indication:
- Renal cell carcinoma
- Thyroid cancer
- Target: FLT3
- FDA Approved Indication:
- acute myeloid leukemia (FLT3+)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Squamous non-small cell lung cancer
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2 overexpressed/amplified)
- Target: PARP
- FDA Approved Indication:
- Ovarian cancer
- Fallopian tube cancer
- Peritoneal cancer
- Target: PD-1
- FDA Approved Indication:
- Colorectal cancer (dMMR and MSI-H)
- Head and neck squamous cell carcinoma
- Hepatocellular carcinoma
- Hodgkin lymphoma
- Melanoma
- Non-small cell lung cancer
- Renal cell carcinoma
- Urothelial carcinoma
- Target: PARP
- FDA Approved Indication:
- Ovarian cancer (with BRCA mutation)
- Target: PDGFRα
- FDA Approved Indication:
- Soft tissue sarcoma
- Target: EGFR
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR T790M mutation)
- Target: CDK4, CDK6
- FDA Approved Indication:
- Breast cancer (HR+, HER2-)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Colorectal cancer (KRAS wild type)
- Target: VEGFR, PDGFR, KIT
- FDA Approved Indication:
- Renal cell carcinoma
- Target: PD-1
- FDA Approved Indication:
- Classical Hodgkin lymphoma
- Colorectal cancer (MSI-H/dMMR)
- Gastric cancer
- Melanoma
- Non-small cell lung cancer (PD-L1+)
- Head and neck squamous cell carcinoma
- Urothelial cancer
- Solid tumors (MSI-H/dMMR)
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Target: VEGFR2
- FDA Approved Indications:
- Colorectal cancer
- Gastric cancer or Gastroesophageal junction (GEJ) adenocarcinoma
- Non-small cell lung cancer
- Target: KIT, PDGFRβ, RAF, RET, VEGFR1/2/3
- FDA Approved Indications:
- Colorectal cancer
- Gastrointestinal stromal tumors
- Hepatocellular carcinoma
- Target: CDK4, CDK6
- FDA Approved Indication:
- Breast cancer (HR+, HER2-)
- Target: PARP
- FDA Approved Indication:
- Ovarian cancer (with BRCA mutation)
- FDA Approved Indication:
- Prostate cancer
- FDA Approved Indication:
- Basal cell carcinoma
- Target: VEGFR, PDGFR, KIT, RAF
- FDA Approved Indication:
- Hepatocellular carcinoma
- Renal cell carcinoma
- Thyroid carcinoma
- Target: mTOR
- FDA Approved Indication:
- Renal cell carcinoma
- Target: MEK
- FDA Approved Indication:
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Gastric cancer (HER2+)
- Target: EGFR (HER1/ERBB1), RET, VEGFR2
- FDA Approved Indication:
- Medullary thyroid cancer
- Target: BRAF
- FDA Approved Indication:
- Melanoma (with BRAF V600 mutation)
- Target: PTCH, Smoothened
- FDA Approved Indication:
- Basal cell carcinoma
- Target: PIGF, VEGFA/B
- FDA Approved Indication:
- Colorectal cancer
DRUGS FOR LEUKEMIA AND LYMPHOMA
- Target: CD20
- FDA Approved Indications:
- Non-Hodgkin’s lymphoma
- Chronic lymphocytic leukemia
- Rheumatoid arthritis
- Granulomatosis with polyangiitis
- Target:CD20
- FDA Approved Indications:
- Chronic lymphocytic leukemia
- Diffuse large B-cell lymphoma
- Follicular lymphoma
- Target: HDAC
- FDA Approved Indication:
- Cutaneous T-cell lymphoma
- Peripheral T-cell lymphoma
- Target: HDAC
- FDA Approved Indication:
- Peripheral T-cell lymphoma
- Target: CD20
- FDA Approved Indication:
- Chronic lymphocytic leukemia
- Target: CD19/CD3
- FDA Approved Indication:
- Acute lymphoblastic leukemia (precursor B-cell)
- Target: ABL
- FDA Approved Indication:
- Chronic myelogenous leukemia (Philadelphia chromosome positive)
- Target: CD20
- FDA Approved Indication:
- Chronic lymphocytic leukemia
- Follicular lymphoma
- Target: ABL
- FDA Approved Indication:
- Chronic myelogenous leukemia (Philadelphia chromosome positive)
- Acute lymphoblastic leukemia (Philadelphia chromosome positive)
- Target: BCL2
- FDA Approved Indication:
- Chronic lymphocytic leukemia (with 17p deletion)
- Target: HDAC
- FDA Approved Indication:
- Cutaneous T-cell lymphoma
- Target: CD20
- FDA Approved Indication:
- Non-Hodgkin's lymphoma
- Target: IL-6
- FDA Approved Indication:
- Multicentric Castleman's disease
- Target: JAK1/2
- FDA Approved Indication:
- Myelofibrosis
- TARGET: CD52
- FDA Approved Indication:
- B-cell chronic lymphocytic leukemia
- Target: ABL, FGFR1-3, FLT3, VEGFR2
- FDA Approved Indication:
- Chronic myelogenous leukemia
- Acute lymphoblastic leukemia (Philadelphia chromosome positive)
- Target: HDAC
- FDA Approved Indication:
- Multiple myeloma
Elotuzumab (Empliciti)
- Target: SLAMF7 (CS1/CD319/CRACC)
- FDA Approved Indication:
- Multiple myeloma
- Target: CD38
- FDA Approved Indication:
- Multiple myeloma
- Target: Proteasome
- FDA Approved Indication:
- Multiple myeloma
- Target:Proteasome
- FDA Approved Indication:
- Multiple myeloma,
- Mantle cell lymphoma
- Target:CD30
- FDA Approved Indication:
- Hodgkin lymphoma
- Anaplastic large cell lymphoma
- Target: CD20
- FDA Approved Indication:
- Non-Hodgkin's lymphoma