Translate:
We Can Detect Cancer with a Blood Test: Important in Diagnosis & Treatment of "Lung & Colon Cancer"
What is Precision Cancer Treatment ?
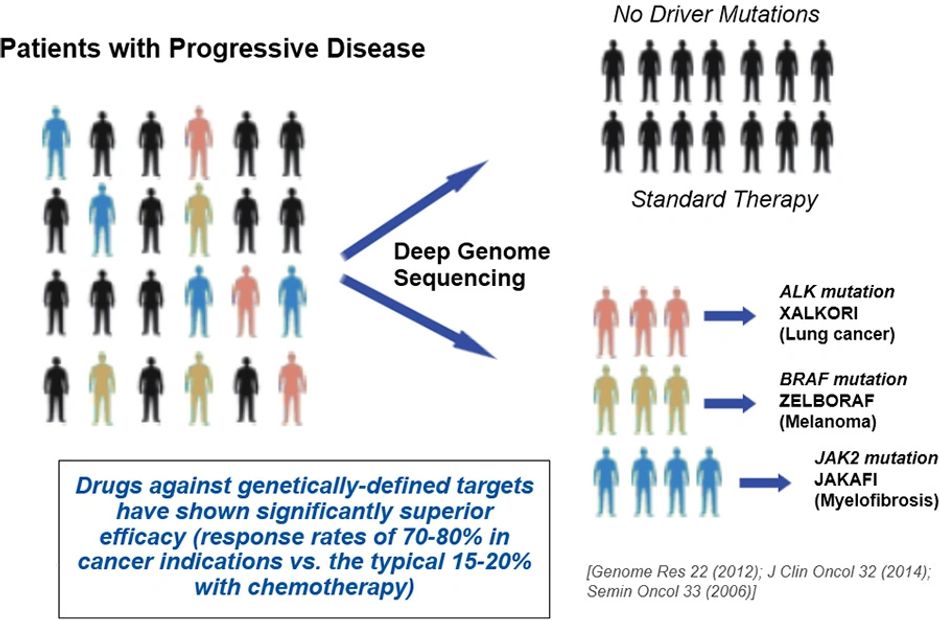
Precision Medicine in Cancer Treatment
Precision medicine is an approach to patient care that allows doctors to select treatments that are most likely to help patients based on a genetic understanding of their disease.
Changes that occur in one person’s cancer may not occur in others who have the same type of cancer.
The same cancer-causing changes may be found in different types of cancer.
To be eligible for precision medicine, your tumor must be tested for genetic change that can be targeted by a treatment currently available in the market or in Clinical Trials.
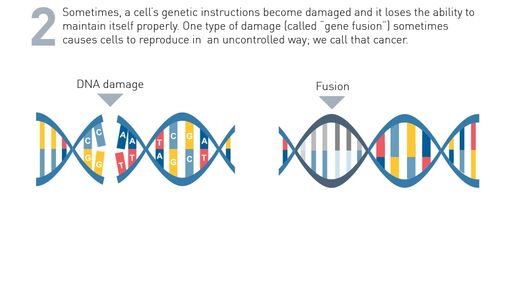
How Genetic Changes in Your Cancer Are Identified
To figure out which genetic changes are in your cancer, you may need to have a biopsy.
A biopsy is a procedure in which your doctor removes a sample of the cancer.
Your sample will be sent to the Star Biosciences Lab, where a machine called a DNA sequencer looks for genetic changes that may be causing the cancer to grow.
The process of looking for genetic changes in cancer may be called DNA sequencing, genomic testing, molecular profiling, or tumor profiling.

Discuss Precision Medicine Options with your Oncologist
Contact Star Biosciences to Begin your Precision Treatment Journey
Repertoire of Precision Tests to Detect Cancer Biomarkers
Immuno - Oncology
Immuno-Oncology (I-O) is the investigation of innovative approaches that aim to harness the body’s natural immune response to fight cancer.
Immuno-Oncology testing helps determine which patients are good candidates for immunotherapy. Star Bioscience Laboratories is a leader in precision diagnostics and is at the forefront of immunotherapy testing.
Tumor Mutation Burden (TMB): This genomic biomarker is designed to predict response to checkpoint inhibitor immunotherapies targeting the PD-1 and PD-L1 proteins. Studies in lung, melanoma and bladder cancers showed that objective response to checkpoint immunotherapy was predicted by the presence of high TMB.
Microsatellite Instability (MSI) and Mismatch Repair (MMR): KEYTRUDA® was approved by the FDA for the treatment of certain patients with metastatic solid tumors that have been identified as being MSI-high or mismatch repair deficient (dMMR)1. OPDIVO® is FDA-approved for certain colorectal cancer patients with MSI-high or dMMR2.
PD-L1: This immunohistochemical assay detects the level and expression sites of PD-L1 protein. As a companion or complementary diagnostic for certain tumors and indications, it helps identify patients for treatments including KEYTRUDA, OPDIVO, TECENTRIQ® and IMFINZI™.
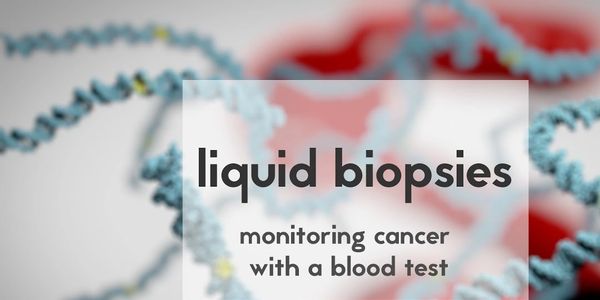
Liquid Biopsy
Its a "Blood Test". It helps your doctor identify whether your treatment is working and pinpoints the status of your disease progression. Tests are highly specific for Lung, Breast, Colon and Prostate Cancers. Test results are delivered within 5 days.
Our Liquid Biopsy services can assist our patients in:
- Performing tumor profiling
- Surveillance of tumors and their response to therapy
- Monitor heterogeneity
- Testing for minimal residual disease (MRD)
- Helping predict early relapse
Testing using liquid biopsies is more sensitive than other diagnostic testing methods like bone marrow taps, which are invasive and painful for patients.
Multi - Gene Testing
Targeted therapy is a newer type of cancer treatment that uses drugs or other substances to more precisely identify and attack cancer cells. Targeted therapy is a growing part of the treatment for many types of cancer.
Multi-Gene testing helps your doctor identify drugs that work against the "Genetic Defect" causing your Cancer. These tests are performed on "All Cancer Types" and help Personalize your Treatment.
Cancer Specific tests:
- Lung Cancer
- Breast Cancer
- Colon Cancer
- Pancreatic Cancer
- Prostate Cancer
- Glioblastoma
- Sarcoma's
- Melanoma
- Leukemia & Lymphoma's
Star Biosciences brings breadth and depth of service to support oncologists in making diagnoses, assessing prognoses, and discovering opportunities for targeted therapy in their patients with solid tumors.
Hereditary Cancer Screening Tests

Test to Screen 25 Hereditary Cancers
Extended Test to Screen BRCA-1 BRCA-2
Saliva-Blood Based Screening
Screens BRCA1, BRCA2 & 21 additional Genes for
Genetic Counseling Included
Contact Us
India: Contact Customer Service Representatives @ Tel: 9794048252
SE-Asia:
Drop us a line and our Customer Service will contact you.
Africa:
Drop us a line and our Customer Service will contact you.
FDA Approved Precision Drugs for Cancer

DRUGS FOR SOLID TUMORS
Ado-trastuzumab emtansine (Kadcyla)
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Target: EGFR (HER1/ERBB1), HER2 (ERBB2/neu)
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR exon 19 deletions or exon 21 substitution (L858R) mutations)
- FDA Approved Indication:
- Renal cell carcinoma
- Melanoma.
- Target: ALK
- FDA Approved Indication:
- Non-small cell lung cancer (with ALK fusion)
- Target: PD-L1
- FDA Approved Indication:
- Urothelial carcinoma
- Non-small cell lung cancer
- Target: PD-L1
- FDA Approved Indication:
- Merkel cell carcinoma
- Urothelial cancer
- Target: KIT, PDGFRβ, VEGFR1/2/3
- FDA Approved Indication:
- Renal cell carcinoma
- Target: BAFF
- FDA Approved Indication:
- Lupus erythematosus
- Target: VEGF ligand
- FDA Approved Indications:
- Cervical cancer
- Colorectal cancer
- Fallopian tube cancer
- Glioblastoma
- Non-small cell lung cancer
- Ovarian cancer
- Peritoneal cancer
- Renal cell carcinoma
- Target: ABL
- FDA Approved Indication:
- Chronic myelogenous leukemia (Philadelphia chromosomepositive)
- Target:ALK
- FDA Approved Indication:
- Non-small cell lung cancer (ALK+)
Cabozantinib (Cabometyx [tablet], Cometriq [capsule])
- Target: FLT3, KIT, MET, RET, VEGFR2
- FDA Approved Indication:
- Medullary thyroid cancer
- Renal cell carcinoma
- Target: ALK
- FDA Approved Indication:
- Non-small cell lung cancer (with ALK fusion)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Colorectal cancer (KRAS wild type),
- Squamous cell cancer of the head and neck
- Target: MEK
- FDA Approved Indication:
- Melanoma (with BRAF V600E or V600K mutation)
- Target: ALK, MET, ROS1
- FDA Approved Indication:
- Non-small cell lung cancer (with ALK fusion or ROS1 genealteration)
- Target: BRAF
- FDA Approved Indication:
- Target: RANKL
- FDA Approved Indication:
- Giant cell tumor of the bone
- Target: B4GALNT1 (GD2)
- FDA Approved Indication: Pediatric neuroblastoma
- Target: PD-L1
- FDA Approved Indication:
- Urothelial carcinoma
- Non-small cell lung cancer
- Target: IDH2
- FDA Approved Indication:
- Acute myeloid leukemia (with IDH2 mutation)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR exon 19 deletions or exon 21 substitution (L858R) mutations)
- Pancreatic cancer
- Target: mTOR
- FDA Approved Indication:
- Pancreatic, gastrointestinal, or lung origin neuroendocrine tumor
- Renal cell carcinoma
- Nonresectable subependymal giant cell astrocytoma associated with tuberous sclerosis
- Breast cancer (HR+, HER2-)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR exon 19 deletions or exon 21 substitution (L858R) mutations)
- Target: BTK
- FDA Approved Indication:
- Mantle cell lymphoma
- Chronic lymphocytic leukemia
- Waldenstrom's macroglobulinemia
- Target: PI3Kδ
- FDA Approved Indication:
- Chronic lymphocytic leukemia
- Follicular B-cell non-Hodgkin lymphoma
- Small lymphocytic lymphoma
- Target: KIT, PDGFR, ABL
- FDA Approved Indication:
- GI stromal tumor (KIT+)
- Dermatofibrosarcoma protuberans
- Multiple hematologic malignancies including Philadelphia chromosome-positive ALL and CML
- Target: CTLA-4
- FDA Approved Indication:
- Melanoma
- Renal cell carcinoma
- Target: Proteasome
- FDA Approved Indication: Multiple Myeloma
- Target: HER2 (ERBB2/neu), EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Target: VEGFR2
- FDA Approved Indication:
- Renal cell carcinoma
- Thyroid cancer
- Target: FLT3
- FDA Approved Indication:
- acute myeloid leukemia (FLT3+)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Squamous non-small cell lung cancer
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2 overexpressed/amplified)
- Target: PARP
- FDA Approved Indication:
- Ovarian cancer
- Fallopian tube cancer
- Peritoneal cancer
- Target: PD-1
- FDA Approved Indication:
- Colorectal cancer (dMMR and MSI-H)
- Head and neck squamous cell carcinoma
- Hepatocellular carcinoma
- Hodgkin lymphoma
- Melanoma
- Non-small cell lung cancer
- Renal cell carcinoma
- Urothelial carcinoma
- Target: PARP
- FDA Approved Indication:
- Ovarian cancer (with BRCA mutation)
- Target: PDGFRα
- FDA Approved Indication:
- Soft tissue sarcoma
- Target: EGFR
- FDA Approved Indication:
- Non-small cell lung cancer (with EGFR T790M mutation)
- Target: CDK4, CDK6
- FDA Approved Indication:
- Breast cancer (HR+, HER2-)
- Target: EGFR (HER1/ERBB1)
- FDA Approved Indication:
- Colorectal cancer (KRAS wild type)
- Target: VEGFR, PDGFR, KIT
- FDA Approved Indication:
- Renal cell carcinoma
- Target: PD-1
- FDA Approved Indication:
- Classical Hodgkin lymphoma
- Colorectal cancer (MSI-H/dMMR)
- Gastric cancer
- Melanoma
- Non-small cell lung cancer (PD-L1+)
- Head and neck squamous cell carcinoma
- Urothelial cancer
- Solid tumors (MSI-H/dMMR)
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Target: VEGFR2
- FDA Approved Indications:
- Colorectal cancer
- Gastric cancer or Gastroesophageal junction (GEJ) adenocarcinoma
- Non-small cell lung cancer
- Target: KIT, PDGFRβ, RAF, RET, VEGFR1/2/3
- FDA Approved Indications:
- Colorectal cancer
- Gastrointestinal stromal tumors
- Hepatocellular carcinoma
- Target: CDK4, CDK6
- FDA Approved Indication:
- Breast cancer (HR+, HER2-)
- Target: PARP
- FDA Approved Indication:
- Ovarian cancer (with BRCA mutation)
- FDA Approved Indication:
- Prostate cancer
- FDA Approved Indication:
- Basal cell carcinoma
- Target: VEGFR, PDGFR, KIT, RAF
- FDA Approved Indication:
- Hepatocellular carcinoma
- Renal cell carcinoma
- Thyroid carcinoma
- Target: mTOR
- FDA Approved Indication:
- Renal cell carcinoma
- Target: MEK
- FDA Approved Indication:
- Target: HER2 (ERBB2/neu)
- FDA Approved Indication:
- Breast cancer (HER2+)
- Gastric cancer (HER2+)
- Target: EGFR (HER1/ERBB1), RET, VEGFR2
- FDA Approved Indication:
- Medullary thyroid cancer
- Target: BRAF
- FDA Approved Indication:
- Melanoma (with BRAF V600 mutation)
- Target: PTCH, Smoothened
- FDA Approved Indication:
- Basal cell carcinoma
- Target: PIGF, VEGFA/B
- FDA Approved Indication:
- Colorectal cancer
DRUGS FOR LEUKEMIA AND LYMPHOMA
- Target: CD20
- FDA Approved Indications:
- Non-Hodgkin’s lymphoma
- Chronic lymphocytic leukemia
- Rheumatoid arthritis
- Granulomatosis with polyangiitis
Rituximab/hyaluronidase human (Rituxan Hycela)
- Target:CD20
- FDA Approved Indications:
- Chronic lymphocytic leukemia
- Diffuse large B-cell lymphoma
- Follicular lymphoma
- Target: HDAC
- FDA Approved Indication:
- Cutaneous T-cell lymphoma
- Peripheral T-cell lymphoma
- Target: HDAC
- FDA Approved Indication:
- Peripheral T-cell lymphoma
Ofatumumab (Arzerra, HuMax-CD20)
- Target: CD20
- FDA Approved Indication:
- Chronic lymphocytic leukemia
- Target: CD19/CD3
- FDA Approved Indication:
- Acute lymphoblastic leukemia (precursor B-cell)
- Target: ABL
- FDA Approved Indication:
- Chronic myelogenous leukemia (Philadelphia chromosome positive)
- Target: CD20
- FDA Approved Indication:
- Chronic lymphocytic leukemia
- Follicular lymphoma
- Target: ABL
- FDA Approved Indication:
- Chronic myelogenous leukemia (Philadelphia chromosome positive)
- Acute lymphoblastic leukemia (Philadelphia chromosome positive)
- Target: BCL2
- FDA Approved Indication:
- Chronic lymphocytic leukemia (with 17p deletion)
- Target: HDAC
- FDA Approved Indication:
- Cutaneous T-cell lymphoma
- Target: CD20
- FDA Approved Indication:
- Non-Hodgkin's lymphoma
- Target: IL-6
- FDA Approved Indication:
- Multicentric Castleman's disease
- Target: JAK1/2
- FDA Approved Indication:
- Myelofibrosis
- TARGET: CD52
- FDA Approved Indication:
- B-cell chronic lymphocytic leukemia
- Target: ABL, FGFR1-3, FLT3, VEGFR2
- FDA Approved Indication:
- Chronic myelogenous leukemia
- Acute lymphoblastic leukemia (Philadelphia chromosome positive)
- Target: HDAC
- FDA Approved Indication:
- Multiple myeloma
Elotuzumab (Empliciti)
- Target: SLAMF7 (CS1/CD319/CRACC)
- FDA Approved Indication:
- Multiple myeloma
- Target: CD38
- FDA Approved Indication:
- Multiple myeloma
- Target: Proteasome
- FDA Approved Indication:
- Multiple myeloma
- Target:Proteasome
- FDA Approved Indication:
- Multiple myeloma,
- Mantle cell lymphoma
Brentuximab vedotin (Adcetris)
- Target:CD30
- FDA Approved Indication:
- Hodgkin lymphoma
- Anaplastic large cell lymphoma
Ibritumomab tiuxetan (Zevalin)
- Target: CD20
- FDA Approved Indication:
- Non-Hodgkin's lymphoma